Linamar Announces Creation of Linamar MedTech, a New Group Focused on Medical Devices and Precision Medical Components

May 20, 2022
Linamar Corporation recently announced the formation of Linamar MedTech, a new medical manufacturing group which will focus on leveraging its expert capabilities in precision manufacturing to pursue opportunities in the Medical Device and Precision Medical Component markets.
Linamar MedTech will further fuel growth and diversification in line with the company’s 2100 strategy. It demonstrates Linamar’s commitment and belief in future opportunities within the medical devices industry. The announcement builds on the medical device programs the company has launched over the past several years, including full ventilator systems and ventilator parts, part of the highly critical, expeditated launch effort in response to the public health crisis created by the COVID-19 pandemic. Linamar has also successfully launched other medical device programs in automated robotic microscopy.
“Linamar has expertise and resources that are applicable beyond the markets in which it operates today as we have proven with prior diversification initiatives,” said Linda Hasenfratz, Linamar’s Executive Chair and CEO. “This announcement formalizes the creation of Linamar MedTech, a new group with a dedicated team to pursue medical device and precision medical component business opportunities. With a growing and aging population, the medical device and precision components market is one that is growing quickly. This is an exciting new area for Linamar to grow, and one in which we have already proven we can excel.”
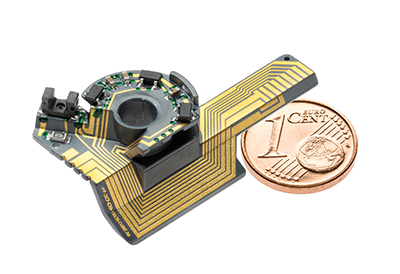
Linamar’s quality management system has demonstrated it can meet the required industry standards for medical devices. By utilizing the Linamar global operating structure and manufacturing footprint, Linamar plans to manufacture precision medical components and complex medical device assemblies for the global medical market. Potential targeted products include surgical, respiratory, and imaging devices and precision components for devices, orthopedics, and prosthesis.
“Linamar has demonstrated we can supply this market with high-quality products at competitive prices,” said Jim Jarrell, Linamar’s President and COO. “Medical devices and precision medical components are a market where we see we can add immediate value by leveraging our scale and capabilities to bring innovative, high-quality, and cost-effective solutions to customers. Linamar’s capabilities in manufacturing, supply chain and program management can be leveraged to offer medical equipment OEMs advanced contract manufacturing solutions, for better products at a better price. Our history in innovation and lean manufacturing principles points to a strong future for Linamar MedTech to deliver value to our customers and stakeholders.”