How Factory Automation Supports the Medical Device Industry

January 3, 2025
Medical devices are subject to strict regulations and high-quality control standards. Automation can help.
The healthcare industry is among the most highly advanced manufacturing fields in the world, with tight controls and restrictions governing the development and production of medical devices at every stage. At the same time, researchers and developers in the healthcare industry benefit from manufacturing solutions that are flexible, reliable, and innovative.
The tension in the healthcare industry between innovation and regulation has historically been difficult to balance. However, factory automation offers a resolution to some of these competing priorities by helping medical device manufacturers produce cutting-edge products that meet even the most stringent industry requirements. Here’s how:
1. Automation offers advanced quality control measures
Quality control is important in every industry, but in healthcare, the consequences of a flawed device can be dire. As such, advanced quality control measures are even more important.
Automation already directly benefits production quality by enhancing standardization and removing opportunities for human error. A human worker on an assembly line can only be so consistent. Some inconsistencies may not be significant enough to matter, but others might be flaws in themselves or lead to flaws further down the production line. Automation is not only more consistent than manual production but can be optimized to a more precise tolerance level than what human workers can achieve.
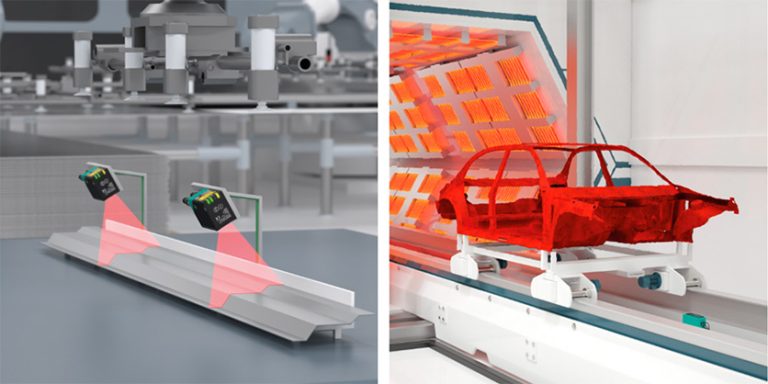
Given the quality benefits of automation in the manufacturing process, it’s only natural to apply these same processes to testing and inspection. Traditional quality control measures have used mistake-proofing techniques to prevent defects as they occur. Modern methods, such as vision technology and advanced sensors, expand the range of quality testing a company can incorporate directly into the production lines.
2. Advanced robotics help manufacturers keep their operations flexible
Stationary automated assembly lines have long been the standard for many high-volume manufacturers, but the downside of these assemblies is that the specialized equipment cannot easily be switched over to new uses if a production technique changes, or if market demand suddenly shifts. Given the myriad supply chain disruptions industries around the world have faced since the onset of the Covid-19 pandemic, manufacturing flexibility is a growing priority in some sectors.
Modern robots offer a compelling solution. With a range of effectors that can be rapidly switched during an assembly, a single robot can perform numerous operations repeatedly and consistently. Programming robots has also become more user-friendly, with some interfaces allowing an operator to easily reprogram a robot with the push of a few buttons.
This means that robots are now able to work alongside human operators, assisting in routine tasks both on the factory floor, and in other work settings.
3. Manufacturing consistency helps devices meet FDA approval
While flexibility around production can help manufacturers change gears in a pinch, consistency also has its benefits. Medical devices must meet various levels of FDA approval, with entirely novel devices requiring a higher standard and longer approval process than those that are similar to devices already being produced.
If a proven production process is used to produce components of a new device, this similarity works in the manufacturer’s favor. In this way, a consistent production process not only means more reliable quality control but also a faster approval process.
4. High-mix, low-volume (HMLV) production allows for customization
While some medical devices are suited for production by the millions, others are complex enough that a manufacturer may only receive orders in the hundreds or thousands. For these devices, which have a high variety of components, manufacturers benefit from sophisticated automated solutions, such as those that can be provided by the latest high-end robots.
Similarly, some medical devices need to be customized for the patient. These are the kinds of extremely low-volume products that have typically eluded automation.
5. Production tracking helps businesses meet compliance standards
Some medical devices require manufacturers to be able to trace the production process of individual components through each stage of the automated assembly line. This is especially true for a device that might be implanted within a human body, like a pacemaker. If there ends up being a fault with the device, then it is essential that this flaw can be traced back to its point of origin, so that any other faulty devices can be identified.
Factory automation will fuel advances in the medical device technology of the future.
Automation has already revolutionized the medical device industry, but Industry 4.0 promises to bring even more change. Advanced simulations will give device manufacturers innovative ways to model their assemblies; IIoT and Big Data will provide new tracking tools and deeper insights into production methods; and augmented reality will transform how operators interact with equipment.